- Homepage
- Aspect Ratio
- Brand
- Max. Resolution
- 1280 X 1024 (2)
- 1366 X 768 (8)
- 1366x768 (2)
- 1920 X 1080 (24)
- 1920x1080 (70)
- 1920x1080 Only (8)
- 1920x1200 (2)
- 1920×1080 (3)
- 2160 X 1440 Full Hd (2)
- 2256x1504 (6)
- 2560 X 1600 (5)
- 2736 X 1824 (9)
- 2736 X 1824 Pixels (2)
- 2736x1824 (4)
- 3200 X 1800 (3)
- 3200 X1800 ( Qhd +) (2)
- 3840x2160 ( Uhd ) (3)
- 4800 X 1200 Dpi (2)
- Fhd (2)
- Only For 1920x1080 (3)
- Other (3464)
- Screen Size
- Type
- Button(s) (15)
- Display: Lcd Screen (392)
- Display: Lens Screen (15)
- Display: Oled Screen (11)
- Frame (12)
- Headrest (11)
- Helmet / Action (13)
- Lcd (20)
- Lcd Assembly (15)
- Lcd Display (19)
- Lcd Display Screen (15)
- Lcd Screen (25)
- Notebook / Laptop (24)
- Screen Assembly (21)
- Screen Digitizer (88)
- Screens & Parts (18)
- Sim-free Smartphone (16)
- Single Screen (12)
- Tablet (165)
- Touchscreen (61)
- Other (2658)
- Unit Type
CE+, Portable Ultrasound Scanner ultrasound diagnostic system LCD CMS600B3 USB
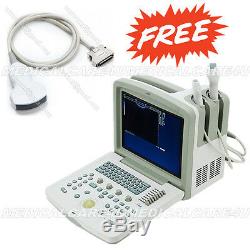










CE+, Portable Ultrasound Scanner ultrasound diagnostic system LCD CMS600B3 USB. Contec Medical Systems---CMS600B3 Ultrasound Scanner.
CMS600B3 is a portable, LCD screen B-ultrasound diagnostic system. It adopts advanced full digital imaging technologies. The device is suitable for ultrasonic examination on abdominal, obstetrics, gynecology, cardiology, small parts, urology.
Adoption of folded backlit keyboard and trackball provides fast, convenient and flexible operation. The image of the device is clear, stable and high resolution.
It has two probe interfaces, and many probes can be chosen, which can satisfy the clinic diagnostic need fully. PAL-D video output offers connection to external video printer and big display and other equipments. High speed USB port provides real-time image transfer to the PC. The system can process image save, zoom, up and down flip, left and right flip, black and white flip, and large-capacity cine loop; multi-level scanning depth, dynamic range, acoustic power, frame correlation, focus number and position etc.
It can generate report automatically according to different the type of measurement. Display mode: B, 2B, 4B, BM, M, BM/M. Focus: The number and position can be adjustable. Image processing: Up and down flip, left and right flip, black and white flip, frame correlation, gamma correction, histogram, image smoothing etc.Character display: Date, time, name, sex, age, doctor, hospital, probe information, image information etc. Measurement: Distance, circumference, area, volume, heart rate , GA, FW, EDD. 3.5 MHz convex probe. 5.0 MHz micro-convex probe.
6.5 MHz transvaginal probe. 7.5 MHz linear probe. 7.5 MHz transrectal probe. Dimension: 289 mm×304 mm×222 mm Weight: 6.4 kg.
It take 7-20 bussiness days to arrive you. EMS, UPS, TNT and DHL could be used for specail requirment if you agree to pay more. The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923. And certified by FDA of United States and CE, TUV of Europe.
The Fingertip Pulse Oximeter that is FDA 510K Approved. The item "CE+, Portable Ultrasound Scanner ultrasound diagnostic system LCD CMS600B3 USB" is in sale since Tuesday, August 11, 2015. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "medicalcare4u" and is located in Qinhuangdao. This item can be shipped to North, South, or Latin America, all countries in Europe, all countries in continental Asia, Australia.
- Model: CMS600B3
- Country/Region of Manufacture: China
- MPN: 69450401
- Brand: CON-TEC
- UPC: Does not apply

